Supervisory institution:
National Science Centre
Project manager:
Dr. Hanna Piotrowska-Kempisty
Budget:
837 786,00 PLN
Start date:
20.09.2017
Duration:
36 months
Contract number:
UMO-2016/23/D/NZ7/03954
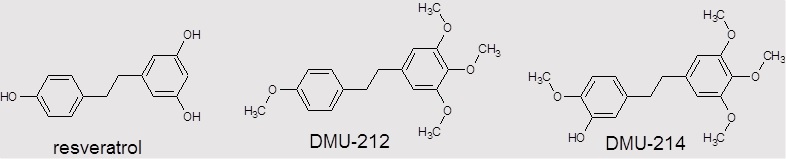
Cancer represents the leading cause of death worldwide, after the cardiovascular diseases. A growing number of cancer cases and serious side effects of first-line chemotherapeutics refer to the high financial and social expenditures. Therefore, there is still a great need to develop studies on effective and safe anti-cancer therapies. A variety of approaches have been considered in anticancer research. Enzymes of CYP1 family such as CYP1A1 and CYP1B1 are frequently overexpressed in a wide range of human cancer cells compared to their normal counterparts. The differential expression of these enzymes within the tumour tissue and the surrounding noncancerous cells offers many opportunities for development of new pro-drugs activated by CYP1A1 and/ or CYP1B1. Our recently published results showed that methylated resveratrol analogue DMU-212 (3,4,5,4’-tetra-methoxystilbene) undergoes metabolic activation to DMU-214 (3’-hydroxy-3,4,5,4’-tetra-methoxystilbene) in ovarian cancer cells since we observed its significantly higher cytotoxic activity as compared to the parent compound. Furthermore, the biological activity of DMU-214 has been observed to be limited mainly to cancer cells.
The main goal of the project is to evaluate the effectiveness of the anti-proliferative activity of the novel DMU-214 in ovarian cancer treatment based on personalized approach. The clinical response to standard chemotherapy differs among patients and biologically-targeted drugs are seldom applied. Such heterogeneous treatment effects and no clinically approved targeted therapeutics represent crucial clinical needs and make it necessary to develop effective personalized treatments for patients with ovarian cancer on the grounds of the molecular and genetic properties of the tumour. The applicants will analyze the anti-proliferative activity of DMU-214 in patient-derived ovarian cancer cells in 3D culture model under normoxia and hypoxia which is characteristic feature of tumour biology. Among a plethora of benefits that the 3D culture holds are: appropriate cell polarity, gene and protein expression levels, interaction between the surrounding cells and the microenvironment. Hence, the proposed by the applicant model refers to as a bridge between flat in vitro and in vivo experimental parts. Finally, in vivo studies using mice bearing patient-derived cancer xenograft will be performed in order to verify the results obtained in in vitro experiments.
As yet there are no data concerning anti-cancer activity of methylated resveratrol analogues, including DMU-214, under hypoxia. Hypoxia is thought to be an adverse prognostic marker in patients with cancer. Our preliminary studies showed, as we expected, that the cytotoxicity of DMU-214 was lower in hypoxia (1% of O2), as compared to normoxia in ovarian cancer cells. Although decreased concentration of oxygen seems to play, to some extent, a negative role in cytotoxicity of DMU-214, its anti-proliferative activity is still significantly higher than that of carboplatin, one of the chemotherapeutics used routinely in ovarian cancer therapy. Since hypoxia has been associated with resistance of cancer cells to front-line chemotherapeutics such as carboplatin, cisplatin, doxorubicin and paclitaxel, further studies concerning the oxygen-dependent mechanism of new potential chemotherapeutics, including DMU-214, should be performed. The gene pathways activated in ovarian cancer which cause chemoresistance as a result of hypoxia are poorly understood. The biochemical and molecular assays (including microarray analyses), which are planned in this project, are the best tools to identify new potential biomarkers of hypoxia and to provide an initial validation of a subset of these markers in ovarian cancer tissues.


