Supervisory institution:
National Science Center
Project manager:
Emerson Coy ,PhD DSc, and Dr. Sergio Moya
Budget:
1 638 220,00 PLN
Start date:
2020-02-27
Duration:
4 years
Contract number:
UMO-2019/33/B/ST5/01495
Nanomedicine represents a niche of opportunities for pharmaceutical companies and biotech industry. Nanocarriers (NCs) offer multiple posibilites for encapsulation of hydrophobic molecules that would otherwise be insoluble, and payloads that have short circulation half-life and/or need to be protected from enzymes in the bloodstream, such as esterases or nucleases. Polymeric NCs have been shown to improve the therapeutic index of drugs by increasing their targeting capacity and accumulation in specific organs, tissues or cells while decreasing potential toxic and off-target side effects. However, the effective transition of the carriers into clinics requires a proper assessment of the interaction of NCs with biomolecules, cells, and in vivo. the kinetics of drug release, in vitro/in vivo degradation, and biological fate and translocation of NCs and NC- drug conjugates.
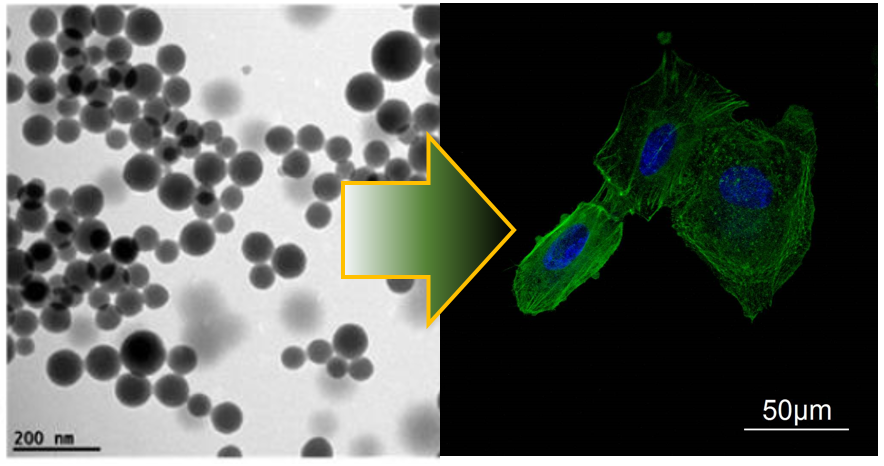
We propose the synthesis and characterization of biocompatible and biodegradable poly lactic-co-glycolic acid (PLGA)-, and polyamine phosphate supramolecular NCs with the aim of investigating their stability, localization, degradation kinetics and biological fate in vitro and in vivo using a combination of imaging modalities. Carries will be engineered for increasing circulation and for specific targetting. Anti TNF alpha antibodies and Silencing RNAs will be assembled on the surface of the PLGA NCs by means of the Layer by Layer (LbL) technique. Silencing RNAs will be as well encapsulated in polyamine phosphate NCs forming a complex with the polyamines. The intracellular localization, aggregation, stability of NC-drug conjugates and mechanism of drug release will be first studied in vitro using a combination of techniques including but not limited to Confocal Laser Scanning Microscopy, Flow Cytometry, Confocal Raman Microscopy and Fluorescence Correlation Spectroscopy.
Protein corona formation and the biological fate of the corona will be studied for the NCs with different surface functionalization. In vitro through Fluorescence Correlation and Fluorescence Correlation Spectroscopy we will investigate the fate of pre formed coronas inside cells and the impact of the coating stealthness on the degree of aggregation of NCs intracelurlaly. Stability of preformed protein corona will be studied in vivo by proper radiolabelling of proteins and NCs.
Radiolabelling of the NCs, ptoteins and/or drugs with positron or gamma emitters will be carried out to enable ultra-sensitive, real-time, in vivo and non-invasive localisation of the NCs/drugs in healthy animals using combined in vivo molecular imaging modalities, including Single Photon Emission Computerised Tomography and Positron Emission Tomography, in order to determine the biological fate of the NCs. Multiple labelling strategies will be implemented to study the stability of the coating and the core of the NCs, as well as the kinetics of drug release, and stability of preformed protein coronas.
The project will advance in the understanding of the complex interaction of polymer NC-drug conjugates with biological matter. Fundamental questions about stability in biological media and drug-delivery mechanisms at cell and body levels will be addressed. The results will be published in top ranked international journals and presented in international scientific conferences.
This project will evaluate the efficacy of nanocarriers to target desired organs, their capacity to deliver conjugated drugs in the targeted organs, and the stability of the coating and the NC itself. This approach is unique and offers novel parameters for the assessment of NCs for medical applications. The tools developed here should: (i) support the development of novel nanomedicines in a faster and safer fashion, contributing to enhance the competitiveness of pharmaceutical companies and biotechs; and (ii) aid in the rational design/production of novel nanomedicines, resulting in new opportunities for the technological sectors.


